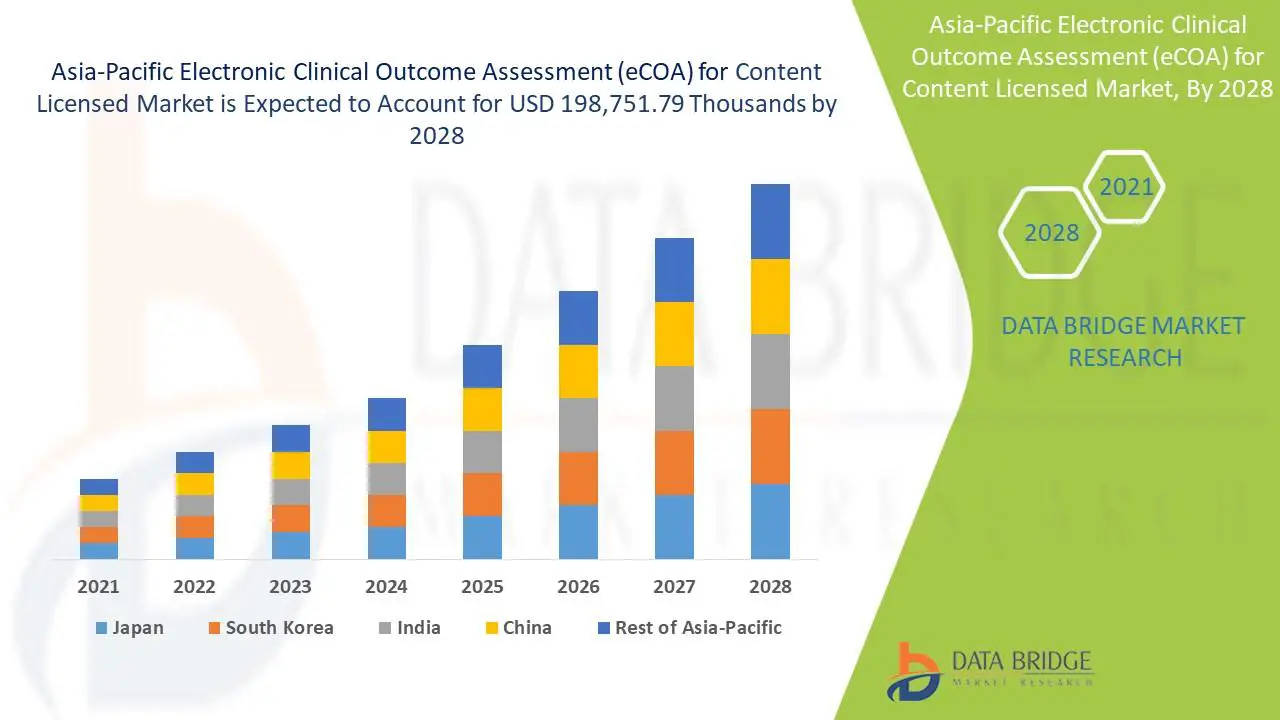
The rise of electronic clinical outcome assessments (eCOAs) in the age of patient centricity which is accelerating the electronic clinical outcome assessment (eCOA) for content licensed market. Electronic clinical outcome assessment (eCOA) is the method of capturing data electronically in clinical trials. It can substantially increase the quality of study data while meeting regulatory requirements. eCOA employs technologies such as handheld devices, tablets or the web to allow trial participants, physicians and caregivers to directly report information related to healthcare outcomes. The electronic clinical outcome assessment (eCOA) for content licensed market is expected to gain market growth in the forecast period of 2021 to 2028. Data Bridge Market Research analyses that the market is growing with the highest CAGR of 15.2% in the forecast period of 2021 to 2028 and expected to reach USD 198,751.79 thousands by 2028.
Get Your Sample Today: https://www.databridgemarketresearch.com/request-a-sample/?dbmr=asia-pacific-electronic-clinical-outcome-assessment-ecoa-for-content-licensed-market
Market Analysis and Insights
For better decisions, more revenue generation, and profitable business, such ASIA-PACIFIC ELECTRONIC CLINICAL OUTCOME ASSESSMENT (ECOA) FOR CONTENT LICENSED market research report is the key. All this data and information is very important to the businesses when it comes to characterize the strategies about the production, marketing, sales, promotion and distribution of the products and services. Growing demand for eCOA due to its capability to collect large amount of data while simultaneously ensuring high quality is accelerating the electronic clinical outcome assessment (eCOA) for content licensed market. This competitive era calls for businesses to be equipped with knowhow of the major happenings of the market and ABC industry. Market segmentation is performed in terms of markets covered, geographic scope, years considered for the study, currency and pricing, research methodology, primary interviews with key opinion leaders, DBMR market position grid, DBMR market challenge matrix, secondary sources, and assumptions.
Buy Now Access Full Report:- https://www.databridgemarketresearch.com/reports/asia-pacific-electronic-clinical-outcome-assessment-ecoa-for-content-licensed-market
Asia-Pacific Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market Scope
· On the basis of approach, the electronic clinical outcome assessment (eCOA) for content licensed market is segmented into clinician reported outcome assessment (ClinRO), patient reported outcome assessment (PRO), observer reported outcome assessment (ObsRO) and performance outcome assessment (PerfO). In 2021, clinician reported outcome assessment (ClinRO) segment holds the largest market share in electronic clinical outcome assessment (eCOA) for content licensed market as ClinRO measure involves a clinical judgment or interpretation of the observable signs, behaviours or other physical manifestations thought to be related to a disease or condition.
· On the basis of end user, the electronic clinical outcome assessment (eCOA) for content licensed market is segmented into commercial service providers, hospitals and transplant centers and research laboratories and academic institutions. In 2021, research laboratories and academic institutions segment holds the largest market share in electronic clinical outcome assessment (eCOA) for content licensed market as research organization and academic institution used broadly within the clinical and drug development industries, primarily refers to an academic and/or non-profit institution that performs one or more functions in the conduct of clinical trials.
· On the basis of platform, the electronic clinical outcome assessment (eCOA) for content licensed market is segmented into contract research organization, pharmaceutical and biopharmaceutical companies, medical device manufacturers, consulting service companies, hospitals and clinical laboratories, research and academia and others. In 2021, contract research organization segment holds the largest market share in electronic clinical outcome assessment (eCOA) for content licensed market as a contract research organization (CROs) is a service organization that provides support to the pharmaceutical and biotechnology industries in the form of outsourced pharmaceutical research services (for both drugs and medical devices).
Browse The Table of Contents And List of Figures: https://www.databridgemarketresearch.com/toc/?dbmr=europe-electronic-clinical-outcome-assessment-ecoa-for-content-licensed-market
Major TOC of the Asia-Pacific Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market Report
Chapter One:
MARKET SEGEMENTATION
EXECUTIVE SUMMARY
PREMIUM INSIGHTS
MARKET OVERVIEW
Asia-Pacific Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market Country Level Analysis
The electronic clinical outcome assessment (eCOA) for content licensed market also provides you with detailed market analysis for every country growth in industry with sales, components sales, impact of technological development in electronic clinical outcome assessment (eCOA) for content licensed and changes in regulatory scenarios with their support for the electronic clinical outcome assessment (eCOA) for content licensed market. The data is available for historic period 2010 to 2019. The countries covered in electronic clinical outcome assessment (eCOA) for content licensed market report are Japan, China, India, South Korea, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, presence and availability of Asia-Pacific brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of sales channels are considered while providing forecast analysis of the country data.
.Competitive Landscape and Asia-Pacific Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market Share Analysis
Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, North America presence, production sites and facilities, company strengths and weaknesses, product launch, product trials pipelines, product approvals, patents, product width and breadth, application dominance, technology lifeline curve. The above data points provided are only related to the companies’ focus related to Asia-Pacific electronic clinical outcome assessment (eCOA) for content licensed market.
The major players covered in the report are
· Oracle,
· IBM Corporation,
· Dassault Systemes,
· Parexel International Corporation,
· ERT Clinical,
· eClinical Solutions LLC,
· ArisGlobal,
· Kayentis,
· Anju Software, Inc.,
· Signant Health,
· WIRB-Copernicus Group and Bioclinica among others local and Asia-Pacific players.
Browse Related Reports@
Europe Plant Breeding and CRISPR Plant Market
Asia-Pacific Plant Breeding and CRISPR Plant Market
Middle East and Africa Plant Breeding and CRISPR Plant Market
North America Plant Breeding and CRISPR Plant Market
Latin America Plant Breeding and CRISPR Plant Market
North America Smart Irrigation Market
About Us:
Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best Market opportunities and foster efficient information for your business to thrive in the Market
Contact:
Data Bridge Market Research
Tel: +1-888-385.85-2818
Email: Sopan.gedam@databridgeMarket research.com